Technical
Sheet
Antisense
Oligonucleotides
Background
Antisense oligonucleotides refer to short, synthetic
oligonucleotides which are complementary in sequence and upon specific
hybridization to its cognate gene product induces inhibition of gene expression.
Oligonucleotides, as short as a 15 mer has the required specificity to inhibit
gene expression of a particular gene by annealing to the cellular mRNA (1,2).
The mechanism of gene expression is based on two properties; the first is the
physical blocking of the translation process by the presence of the short
double stranded region, secondly the presence of the RNA-DNA duplex is
susceptible to cellular RNase H activity. RNase H cleaves the RNA-DNA duplex
region of the mRNA thus preventing the faithful translation of the mRNA (3).
Oligonucleotide Design
The driving force for the search for novel chemical
modification groups compatible with Watson-Crick hybridization of oligonucleotide
was based on the observation of the short stability of naturally occurring
oligonucleotides with phoshodiester bonds. Oligonucleotides with natural
phosphodiester bonds are highly susceptible to rapid degradation by cellular
nucleases. Cellular nucleases have endonuclease activity as well such that 3’
and 5’ end caps are not sufficient to prevent from degradation.
Modification of the phosphodiester bond by replacing one
of the non-bridging oxygen by sulfur imparts resistance to nuclease degradation,
but in general hybridize to the target sequences with lesser affinity than the
phosphodiester counter part.
Internucleotide Linkages & C5 Propyne Analogs
Natural diester linkage, Thioate (S-Oligo) linkage & C5
propyne analogs of dC and dT
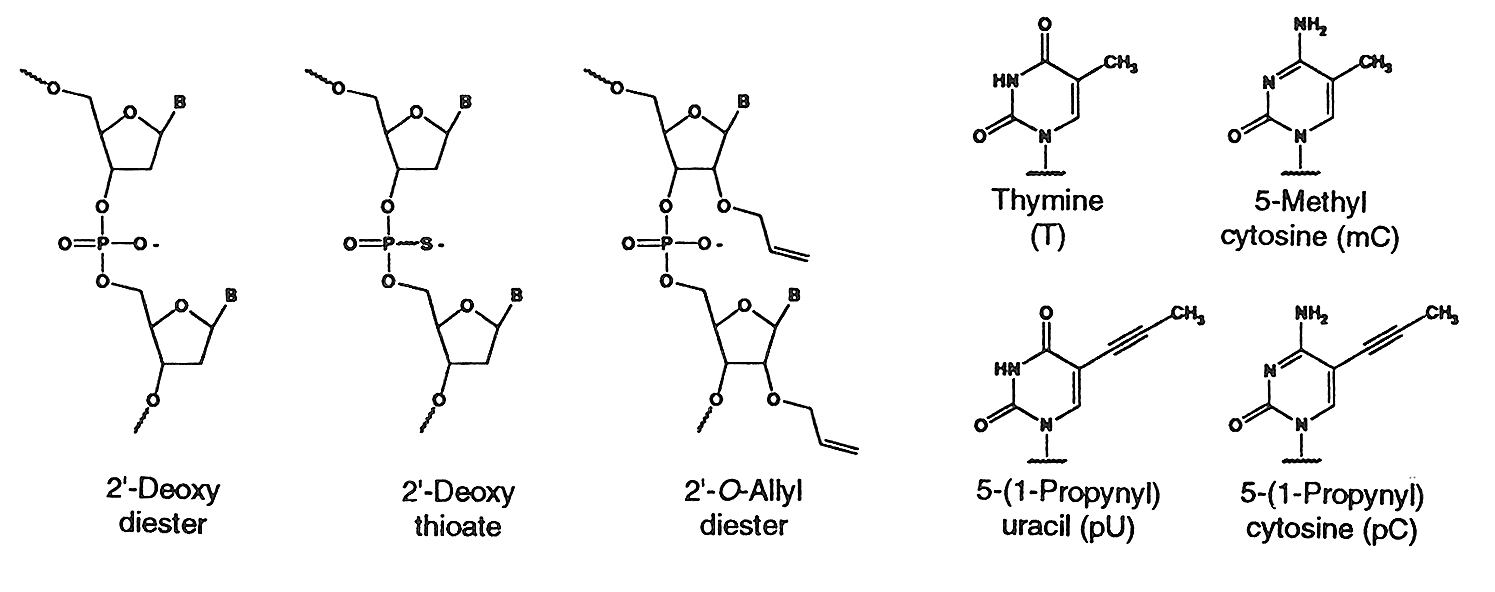
The sulfur substituted oligonucleotides have a
phosphorothiote linkage and are termed as phosphorothioates or simply as
S-oligo. Phosphorothioate oligos are synthesized by Gene Link
using the Beaucage (4)
sulfurizing reagent. The sulfurization reaction is rapid and is performed on
automated DNA synthesizers yielding greater than 96% phosphorothioate linkages,
the remainder are phosphodiester linkages. Custom phosphorothioate oligonucleotides
synthesized by Gene Link can be specified to have all the diester bonds
substituted or only some selected diester linkages depending upon the
researchers experimental requirement. Substitution of all diester linkage is
recommended to provide greater nuclease resistance.
Recently it has been shown that C-5 propyne analogs of dC
and dT when substituted in phosphorothioate oligonucleotide imparts greater
inhibition of gene expression due to increased binding affinity to the target mRNA and increased stability (5).
Based on the above information antisense oligonucleotide
could either be Phosphorothioated at all diester linkages or combined with
substitutions of dC and dT by C-5 propyne analogs pdC and pdU.
References
1. Milligan, J.F.,
Matteucci, M.D. and Martin, J.C. (1993) Current concepts in antisense drug
design. J. Medicinal Chem. 36:1923-1937.
2. Helene, C., Toulme, J.
(1990) Specific regulation of gene expression by antisense, sense and antigene
nucleic acids. Biochim. Biophys. Acta. 1049: 99-125.
3. Weintraub, H. M. (1990)
Antisense RNA and DNA. Sci. Amer. 262:40-46.
4. Iyer, R.P., Egan. W.,
Regan, J.B and Beaucage, S.L. (1990) J. Am. Chem. Soc.112; 1253-1254.
5. Wagner, R.W., Matteucci, M.D.,
Lewis, J.G., Gutierrez, A.J., Moulds, C. and Froehler, B.C. (1993) Antisense
gene inhibition by oligonucleotides containing C-5 propyne pyrimidines. Science
260:1510-1513.
§ § § § § §
Custom Antisense
Oligonucleotide Synthesis
Ordering Information
|
|
Synthesis Scale, $/base NO SET CHARGES |
||||
|
Modification |
200 nmol |
1 m mol |
10 m mol |
15 m mol |
|
|
Phosphorothioate |
$4.25 |
$6.50 |
$50.00 |
$55.00 |
|
Prices subject to change
without notice
All Gene Link products are for research use only.
Please direct all pricing inquiries
to our Orders
department.
For any other problems, please contact our WebMaster.