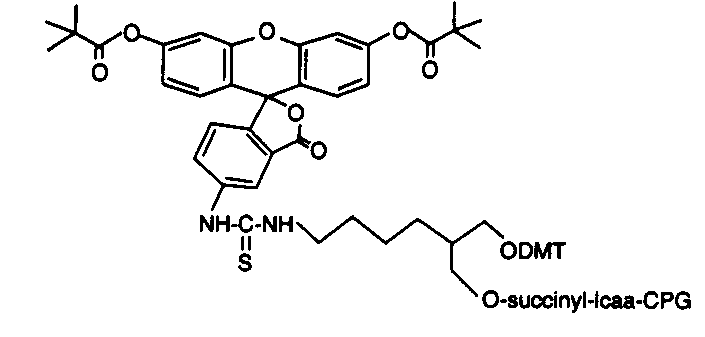
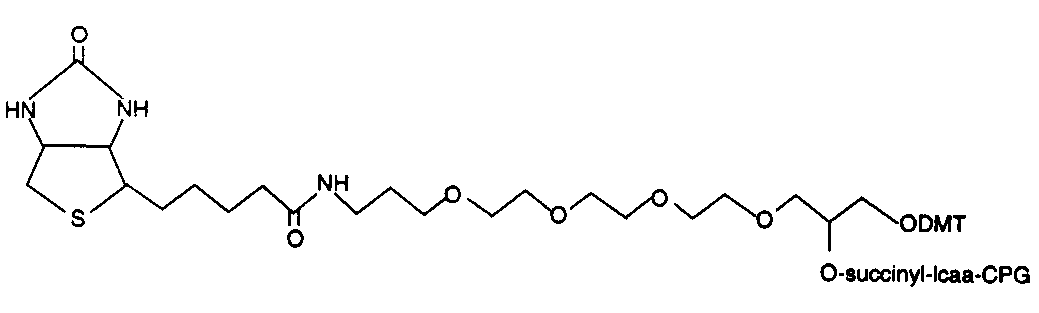
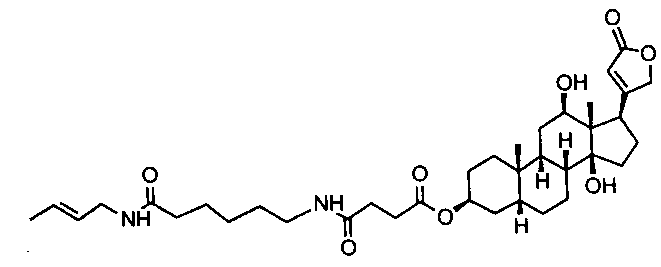
Background
Modification of synthetic oligonucleotides at the 5’ or 3’ position generally is done for either blocking the 5’ or 3’ OH group, for conferring resistance to 3’ exonuclease activity or for further covalent modifications using amine or thiol groups. Other 5’ or 3’ modifications include dye labeling and labeling with biotin and Digoxigenin for non-radioactive detection. Labeling at the 3’ end will block the oligo from chain elongation and thus the primer/oligo cannot be used for elongation in sequencing reaction or for amplification using PCR. Modifications should be done internally (e.g. biotin dT) or at the 5’ end if the oligonucleotide is to used for any polymerase extension.
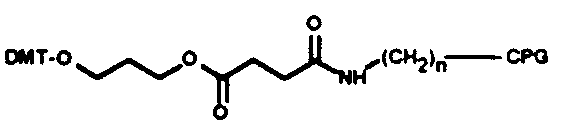
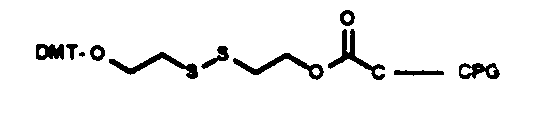
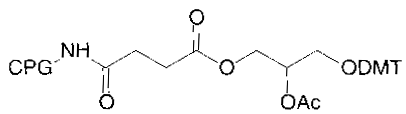
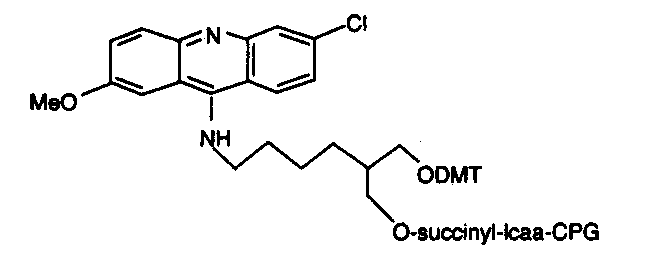
Chain elongation in chemical synthesis of DNA proceeds from 3’ to 5’ orientation, all solid phase automated synthesizers thus use a derivatized matrix as support, generally Controlled Pore Glass (CPG). All 3’ modification thus has the modified group attached to the CPG on which the growing synthetic chain elongates. There is a choice of the spacer arm required between the amino group and the oligonucleotide. This selection is important and is based on the size of the molecule subsequently attached to the oligo via the amino group. A long spacer arm is not required for amino modification of oligonucleotide for subsequent covalent linkage to membranes for hybridization probes as reverse dot blot does, whereas a long spacer arm is desirable for covalent linkage of the oligonucleotide with alkaline phosphatase. The spacer arm modification alone or in combination with an amino group is available in chain lengths of C3, C6, C7, C12 and C27.
Dye labeling
Dye modifications at the 3’ or 5’ end follows the same synthesis mechanism as described above. Gene Link offers dye labels of acridine, fluorescein, rhodamine and others listed in the table, in addition to ABI dyes FAM, HEX, TET for 5’ labeling; and post synthesis 3’ or 5’ labeling for TAMRA and ROX via an amino linker arm.
Purification
Purification of 3’ modified oligonucleotide is strongly recommended as all short truncated sequences will have the 3’ modification and thus may interfere with the detection system involved. This is in contrast to 5’ modification in which only the longest oligonucleotide will contain the modified group. Purification is recommended for all modifications either by Reverse Phase Cartridge (RPC) for oligos shorter than 30 base pairs and by polyacrylamide gel for oligos longer than 30 base pairs. Some modification groups are large aromatic groups e.g. acridine, fluorescein etc. which are hydrophobic and bind strongly to the reverse phase matrix. In general for all modifications the preferred purification method is by polyacrylamide gel.
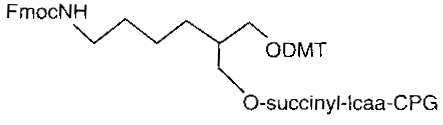
3’ amino C7
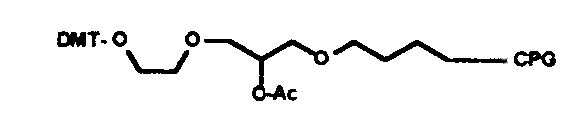
3’ C12 Spacer
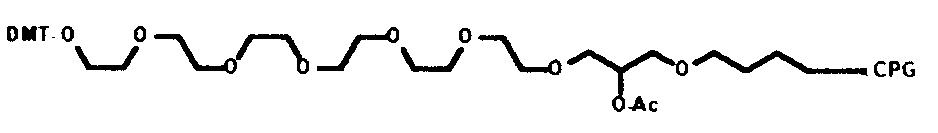
3’ C27 Spacer
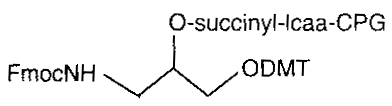
3’ amino C3